views

Intracellular cues, such as damage to the cell's DNA, drive apoptosis primarily through the intrinsic pathway. The intrinsic apoptosis pathway, which involves conserved signaling proteins, is physically associated with mitochondria, and in vertebrates is sensitive to mitochondrial oxidative stress. The pathway is influenced by members of the Bcl family bound to the mitochondrial membrane, including Bax and Bcl-2 gene, which act as pro- or anti-apoptotic regulatory proteins, respectively.
Overview of Intrinsic Apoptosis Pathway
The intrinsic apoptosis pathway is initiated by, for example, chemotherapy and/or radiotherapy. It is activated by a range of exogenous and endogenous stimuli, such as DNA damage, ischemia, and oxidative stress. Moreover, it plays an important function in development and in the elimination of damaged cells.
In the intrinsic pathway, the functional consequence of pro-apoptotic signaling is mitochondrial membrane perturbation and release of cytochrome c in the cytoplasm, where it forms a complex or apoptosome with apoptotic protease activating factor 1 (APAF1) and the inactive form of caspase-9. This complex hydrolyzes adenosine triphosphate to cleave and activate caspase-9. The initiator caspase-9 then cleaves and activates the executioner caspases-3/6/7, resulting in cell apoptosis. It's totally different from the extracellular signals, which are usually generated by cytotoxic cells of the immune system and trigger apoptosis mainly through the extrinsic pathway.
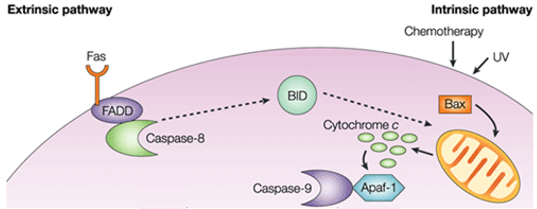
Figure 1. Difference between extrinsic pathway and intrinsic pathway.
Process and Regulation of Intrinsic Apoptosis Pathway
The intrinsic apoptosis pathway induces apoptosis by directly activating caspase-3 or by cleaving bid (BH3 interacting domain death agonist), resulting in mitochondrial dysfunction and subsequent release of cytochrome c and activation of caspases-9 and caspases-3. Caspase-3 promotes the typical apoptosis features, including DNA fragmentation and cell death in several tissues.
The B-cell lymphoma 2 (Bcl-2) protein family tightly controls activation of the intrinsic pathway. It is found in follicular lymphoma and first identified as one of the genes involved in the cell death by either activating pro-apoptotic or inhibiting anti-apoptotic apoptosis. Proteins in one subgroup, including Bid, Bad, Bim, Bmf, Puma, and Noxa, contain a single Bcl-2 homology 3 domain (BH3-only proteins) and have pro-apoptotic activity. Two other protein subsets have multiple BH domains. The first subset, including Bcl-2 associated X protein (Bax), Bcl-2 homologous antagonist/killer (Bak) and Bcl-2 family apoptosis regulator (Bok), is pro-apoptotic; the other subset, including Bcl-2, Bcl-XL, and Mcl-1, is anti-apoptotic. The mitochondrial pathway is partly influenced by Bcl family members bound to the mitochondrial membrane, including both pro-apoptotic regulatory proteins Bax and anti-apoptotic regulatory proteins Bcl-2.
The pro-apoptotic molecules cause permeabilization of the outer mitochondrial membrane, leading to efflux of cytochrome c, which binds the adaptor Apaf-1 and the initiator caspase-9 in the cytosol to form the apoptosome complex. This stimulates caspase-9, which in turn activates the effector caspases. The mitochondrion also releases a protein called Smac/DIABLO into the cytosol. Smac/DIABLO indirectly promotes apoptosis by blocking the effects of a group of anti-apoptotic proteins called inhibitor of apoptosis proteins (IAPs).
The anti-apoptotic proteins Bcl-2 and Bcl-XL inhibit cytochrome c release, whereas Bax, Bak, and Bid, all pro-apoptotic proteins, promote its release from mitochondria. Cytochrome c and deoxyadenosine triphosphate (dATP) bind to APAF-1 to form a multimeric complex that recruits and activates pro-caspase-9, an apoptosis-mediating executioner protease that in turn activates the caspase cascade, resulting in cell apoptosis. During this process, caspase-2, caspase-8, caspase-9 and caspase-10 are involved in the initiation of apoptosis. Caspase-3, caspase-6 and caspase-7 are involved in apoptosis. Caspase-3 and caspase-7 regulate the inhibition of DNA repair and start DNA degradation. In addition, caspase-6 regulates the disintegration of the lamina and cytoskeleton.
Intrinsic Apoptosis Pathway in Pathophysiology
Most chemotherapeutic and targeted cancer therapies kill tumor cells through the generation of pro-death signaling that initiates the intrinsic apoptotic pathway of programmed cell death. The point of no return in the apoptotic cascade is mitochondrial outer membrane permeabilization (MOMP); once it has occurred, mitochondrial permeabilization leads to the formation of an apoptosome, which facilitates caspase activation and subsequently triggers the other hallmarks of apoptotic cell death. The cellular decision to initiate MOMP is controlled by a delicate balance between the pro- and anti-apoptotic molecules of the BCL-2 family.
One of the reasons for chemotherapy resistance is the failure of tumor cells to go into apoptosis due to defects in the intrinsic apoptotic pathway (e.g., changes in p53). Despite significant improvements in treatment, cure rates for many cancers remain suboptimal. The rise of cytotoxic chemotherapy has led to curative therapy for a subset of cancers, though intrinsic treatment resistance is difficult to predict for individual patients. The wave of molecularly targeted therapies has focused on drug gable-activating mutations and is thus limited to specific subsets of patients. The intrinsic mitochondrial pathway of apoptosis represents one promising target for new therapies, and successfully targeting this pathway has the potential to alter the therapeutic landscape of therapy for a variety of cancers.
References:
Loreto, C. The role of intrinsic pathway in apoptosis activation and progression in Peyronie's disease. BioMed Research International. 2014: 61619.
Salvesen, G.S; Duckett, C.S. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002, 3(6): 401-10.





















Comments
0 comment